Accreditation

ISO/IEC 17025:2017 Accredited
Achieving accreditation is a rigorous and thorough process, one that requires dedication and precision. As a newly established lab in 2023, we've committed ourselves to meeting the highest standards. We are proud to be ISO/IEC 17025:2017 Accredited as of Nov 1, 2024. Our active accreditation can be verified at the Perry Johnson Laboratory Accreditation website.
The Lab - Purpose-Built Innovation

Strategic Lab Design for Maximum Sterility
At Core EM Lab, our facility was purposefully designed with meticulous forethought to ensure sterility, safety, and precision at every stage of the testing process. One key example is our negatively pressured receiving room, where all sample shipments are carefully unpacked. This isolated area prevents cross-contamination and ensures that no compromised packaging enters the main lab space, maintaining the integrity of both the samples and the testing environment.
In addition, our molecular room houses all identification equipment in a separate, controlled space to eliminate any risk of interference with other lab processes. This separation allows for more precise testing and ensures that our state-of-the-art identification tools operate under optimal conditions. To further maintain our high standards of cleanliness and safety, all samples are properly disposed of through the biohazard room located at the back of the lab, preventing any exposure or contamination in the working areas.
These intentional design choices reflect our commitment to creating a controlled, sterile environment that exceeds industry standards and ensures the highest level of accuracy and reliability in our testing procedures.
Seeing is Believing: Visual Sample Reporting
At Core EM Lab, we go beyond traditional reporting by incorporating visual evidence directly into our reports. For every sample we process, we take high-resolution images—and, if requested, videos during incubation—to capture the growth of bacteria and fungi in real-time. This unique practice provides our clients with clear, visual validation of microbial growth, offering unmatched transparency in the environmental testing industry.
While other labs may only provide written results, our detailed imagery allows clients to see the extent of contamination firsthand, helping them make more informed decisions. Whether it's a single image of colony formation or a time-lapse video of microbial growth over time, our reports offer a level of clarity and assurance that is simply unparalleled.

State-of-the-Art Microbial Identification Technology
At Core EM Lab, we use the cutting-edge Bruker MALDI Biotyper® (Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight Mass Spectrometry) for microorganism identification. Widely recognized as the gold standard in microbiology, the MALDI Biotyper® provides rapid, highly accurate identification of bacteria, yeast, and fungi. This technology is trusted globally for its unparalleled precision in fields like clinical microbiology, food safety, pharmaceutical testing, and water analysis.
With results delivered in seconds and a reliability rating exceeding 99%, the MALDI-TOF MS ensures that our clients receive precise identification of microorganisms, helping them make informed decisions quickly. By integrating this advanced technology into our workflow, Core EM Lab offers industry-leading identification services that set a new standard for speed and accuracy
Boosting Sterility & Accuracy with BioSafety Cabinets
At Core EM Lab, we integrate the use of biological safety cabinets into our testing process to maximize sterility and reduce the risk of cross-contamination. While achieving 100% sterility and 0% cross-contamination is not feasible, these cabinets play a critical role in minimizing contamination risks, helping to significantly reduce the occurrence of false positives and the need for re-sampling. This additional layer of protection ensures greater accuracy and reliability in our test results, ultimately helping our clients maintain compliance and streamline their operations with fewer disruptions.

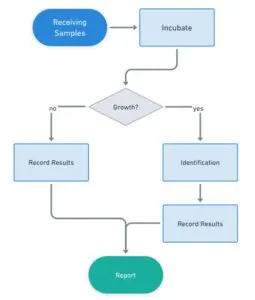
High-Throughput Capabilities
At Core EM Lab, we’ve invested in advanced automation systems that enable us to handle high volumes of samples with unmatched efficiency. Our facility is equipped to incubate over 20,000 plates simultaneously, allowing us to process and analyze samples at scale without compromising accuracy or speed. This capability ensures that we meet our reporting deadlines and guarantees the timely delivery of finalized reports. Whether you're managing routine testing or handling a large influx of samples, our high-throughput infrastructure allows us to deliver consistent, reliable results, keeping your operations running smoothly and on schedule.
Meet The Team

Parthasarathy Chandrakesan MPhil., PhD. Lab Director
Dr. Parthasarathy Chandrakesan is a highly experienced professional with over 20 years in Microbiology and Bioanalytical testing, currently holding a pivotal leadership role at Core EM Lab. He possesses two master’s degrees in Molecular Biology and Endocrinology, along with a PhD in Biomedical Sciences, providing a robust foundation for his expertise across various scientific fields.
In his position at Core EM Lab, Dr. Chandrakesan oversees a diverse range of operations, with a strong emphasis on the importance of timely and precise microbial testing, molecular testing and bioanalytical testing. His dedication to laboratory excellence is evident in his meticulous approach to regulatory compliance, quality assurance, and adherence to GLP, GCP, GMP, ISO, USP, FDA, OSHA, and EPA standards. He has been instrumental in the integration of advanced technologies, including the recent acquisition of a high-throughput machine from Reshape Biotech and Mass Spectrometry from Bruker, significantly enhancing laboratory workflows and high-throughput efficiency.
Dr. Chandrakesan’s leadership and scientific writing skills have been key in establishing the Core EM Lab Quality Manual, as well as the development of Quality Forms and Procedures necessary for maintaining ISO accreditation. His vision for Core EM Lab centers on innovation and integrity, ensuring that the laboratory consistently meets the highest standards of quality and reliability in its services.
Michael Deemer MBA, PMP President, Quality & Safety Manager
As President and Quality Manager of Core EM Lab, Mr. Deemer brings a strong leadership background with a focus on operational excellence and regulatory compliance. With years of experience in business management, contract negotiations, and quality oversight, Mike ensures that Core EM Lab operates at the highest standards of precision and reliability. His commitment to customer service and innovation drives the company forward, delivering customized environmental monitoring solutions that meet the unique needs of each client. Mike's passion for building strong client relationships and implementing efficient processes guarantees that Core EM Lab remains a trusted partner in achieving regulatory compliance and operational success across diverse industries.

